Assignment:
CHEMILUMINESCENCE: SYNTHESIS OF LUMINOL
Q1. Define the following terms.
a. fluorescence
b. phosphorescence
c. chemiluminescence
d. intersystem crossing
Q2. What is the difference in electronic configuration of a singlet and a triplet state?
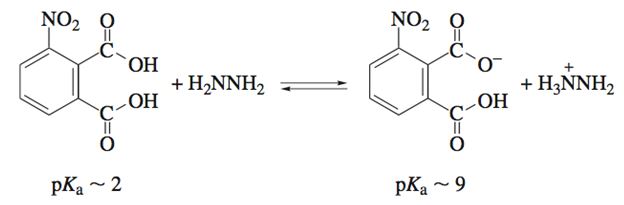
Q3. Given the approximate pKas provided, compute Keq for the reaction below:
ALKYNE FORMATION: DEHYDROBROMINATION OF MESO-STILBENE DIBROMIDE(Preparative)
Q4. The solvent used in the preparation of diphenylacetylene (2) is triethylene glycol.
a. Write the structure of triethylene glycol; circle and label each of the functional groups in this molecule.?
b. What structural features account for the high boiling point of this solvent?
Q5. Give a stepwise mechanism showing the base-induced formation of diphenyl- acetylene from meso-stilbene dibromide. Use curved arrows to symbolize the flow of electrons.
Q6. Why do you think the enthalpy of activation, delta-H ‡, for a syn-periplanar elimination is higher than that for an anti-periplanar elimination?