Assignment:
We can treat a single ion channel as a cylindrical pore through the cell membrane. The channel is of length L = 5 nm and is filled with water. The concentration differences of sodium ions across the membrane is c = 100 mM. This concentration difference produces a flux of ions through the channel, given by Fick's Law. For small ions like sodium, the diffusion constant, D, in water is of the order of 1 µm2/ms.
a. Assuming that the channel cross-section has an area A = 1 nm2, use Fick's Law to calculate the number of sodium ions that pass through the channel every second due to diffusion.
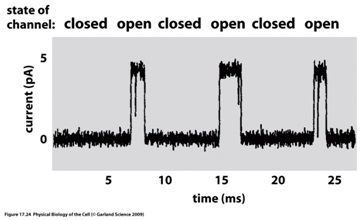
b. Figure shows current recording from a single channel. Use this figure to estimate the number of ions passing through the channel per second, when it is open. Assume that each sodium ion carries an elemental charge, q.
Provide complete and step by step solution for the question and show calculations and use formulas.