Polymers
1. The table below gives intrinsic viscosity values determined by viscometry and weight- average molar mass values determined under the same conditions by static light scattering
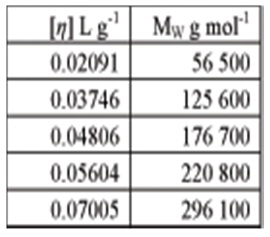
What are the Mark-Houwink parameters for this polymer?
Discuss the applicability of the Mark-Houwink model to this polymer?
2.(a) Given the values below, compute the concentration of benzoyl peroxide needed to prepare polystyrene with a number average molar mass of 400,000 by bulk free radical polymerisation at 60°C, assuming all termination is by combination.
f = 0.6
kd=2×10–6 s–1
kp= 341 M–1 s–1
kt=4×107 M–1s–1
density of styrene = 880 kg m–3.
(b) Compute the initial radical concentration and initial rate of polymerisation for this reaction.
(c) Given the values below for the activation energies of benzoyl peroxide dissociation, styrene propagation, and styrene termination, and assuming all other parameters remain constant compute the number average molar mass, initial radical concentration, and initial rate of polymerisation if the reaction is run at 66°C rather than 60°C.
EA dissociation = 115 kJ mol–1
EA propagation = 34 kJ mol –1
EA termination = 10 kJ mol–1