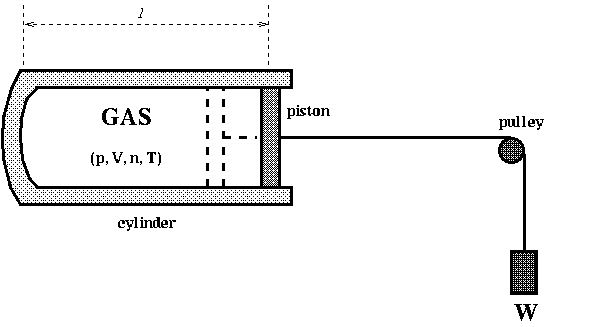
Discuss the below:
Heat and Thermodynamics: Change in internal energy
Q: A cylinder (cross section is 0.2m2) with a free moving piston is filled with gas. The piston is attached to a heavy weight W = 10000N. Outside the cylinder, the air is at 300K and 1 atm. Initially the gas is at 300K, then it is heated to 400K. The heat capacity of the gas under the constant pressure is 500J/K.
If the length of the gas in the cylinder l increases by 20cm during the heating, find the change in the internal energy of the gas in Joule J.