Assignment:
1. Using the result from the previous question, plot the fractions of all the ionization states of glutamic acid, histidine, and lysine as a function of pH, in the pH range of 0-14.
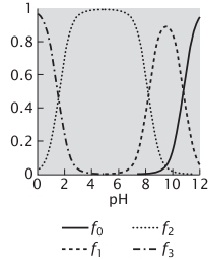
2. Consider the following plot of the ionization of an amino acid. Which amino acid is this?
3. Consider the cis/trans isomerization of a peptide bond. For a bond between two amino acids (different from proline), the equilibrium constant is K = 1000 in favor of trans. If proline is the residue following the peptide bond, K = 4.
(a) What is ΔG° between the two isomers in the two cases above, at room temperature?
(b) Use the partition function to calculate the fraction of cis bonds in both cases.
4. Build short peptides, say a pentapeptide, and set the dihedral angles to each of the values given in Table above, so as to generate all the types of secondary structure and familiarize yourself with these structures.
5. Go to the Kinemage web site (https://kinemage.biochem.duke.eduf). Download and install the program King on your computer. Then consider a central residue (n) and the two flanking residues preceding (n - 1) and following it (n + 1) in a polypeptide chain. For example, designate the peptide carbonyl of the central residue by CO(n), the amide nitrogen group of the following residue by NH(n + 1), and the second carbon of the side chain of the central residue by Cβ(n) (this is also sometimes called Cb or C2). Use the kinemage cl Basics. kin provided at the Kinemage web site, under kinemages from Branden and Tooze (https://kinemage.biochem.duke. edu/kinemage/kinlist.php), or molecular models to explore the Ramachandran diagram. In King, use the measure tool to determine distances between atoms, so that you find out which distances are too short compared to the limits.
(a) The upper right quadrant of the Ramachandran diagram is mainly forbidden because of steric hindrance between which two groups?
(b) What forbidden overlaps block the lower right quadrant of the diagram?
(c) Why is there so much more accessible space on the top left than on the bottom left quadrant? (Hints: think of the differences between glycine and the other amino acids. Use the computer molecular models in this kinemage file or real models to understand the problem.)
(d) The central line at Φ = 0 in the Ramachandran diagram is forbidden mainly because of steric hindrance between which groups?
6. Use the kinemage c2Motifs.kin provided at the Kinemage web site, under kinemages from Branden and Tooze, to do the following:
(a) Do the tutorial for kin 1 (α-helix). Measure the 4) and 4) angles as described in the tutorial for the residues of the a-helix shown there. Record your values and calculate the mean and standard deviation.
(b) Do the same with kin 3 (β-sheet). Again, measure the 4 and 4) angles for strands b2, b3, and b4 for the β-sheet shown. Record your values and calculate the mean and standard deviation.
(c) Produce a Ramachandran diagram: plot the individual values of 4) and 4) that you measured for both the helix and the sheet as points in the Ramachandran plot. Include the plot in your answer.
7. Why does proline break an α-helix at the point where it occurs in the polypeptide chain?
8. Calculate the populations of the electronic states So and Si involved in the absorption of light by tryptophan (λ = 280 nm), at room temperature, using the partition function method. See Section 2.2 for the values of constants
9. Suppose you are studying protein denaturation by the fluorescence emission of a Trp residue of the protein. You obtained a fluorescence spectrum of the protein at 20°C and another at 65°C, and you know that the protein is folded at 20°C and unfolded (denatured) at 65°C. Note that the spectrum of the unfolded protein is red-shifted. Then you recorded a fluorescence spectrum of this protein at 45°C. The three spectra, all normalized to the fluorescence of the highest value recorded, are shown below. The spectrum in gray (45°C) appears intermediate between those at 20°C and 65°C.
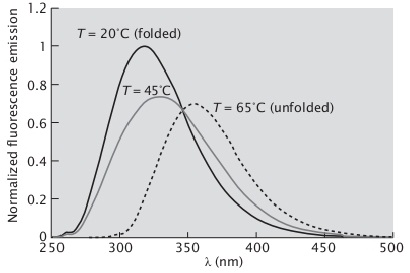
What is the fraction of folded protein at 45°C?
10. Suppose you are following the denaturation of protein A, but now you measure its circular dichroism (CD) spectrum as you increase the temperature. This protein, when folded, is entirely α-helical; when unfolded, it loses all structure (random). The graph below shows the CD spectrum of protein A at a certain temperature T (dashed line) together with the characteristic spectra for an α-helix (solid) and a random coil (dotted). What percent of the proteins are denatured at this temperature?