Solve the following problem:
1. A constant-volume gas thermometer is calibrated in dry ice (-80.0°C ) and in boiling ethyl alcohol (78.0°C ). The respective pressures are 0.900 atm and 1.635 atm. (Note that we have the linear relationship P = A + BT, where A and B are constants.)
(a) What value of absolute zero does the calibration yield?
(b) What pressures would be found at the freezing and boiling points of water? freezing point atm
2. A construction worker uses a steel tape to measure the length of an aluminum support column. If the measured length is 24.700 m when the temperature is 21.2°C, what is the measured length when the temperature rises to 31.4°C? (Note: Do not neglect the expansion of the steel tape. Give your answer to three decimal places.)
3. An air bubble has a volume of 1.80 cm3 when it is released by a submarine 120 m below the surface of a lake. What is the volume of the bubble when it reaches the surface? Assume that the temperature and the number of air molecules in the bubble remains constant during its ascent.
4. A 1.4 m long glass tube that is closed at one end is weighted and lowered to the bottom of a freshwater lake. When the tube is recovered, an indicator mark shows that water rose to within 0.32 m of the closed end. Determine the depth of the lake. Assume constant temperature.
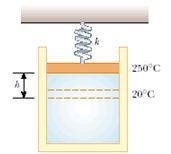
5. An expandable cylinder has its top connected to a spring with force constant 2.00 103 N/m. (See Fig. P10.58.) The cylinder is filled with 5.00 L of gas with the spring relaxed at a pressure of 1.00 atm and a temperature of 20.0°C. (a) If the lid has a cross-sectional area of 0.0100 m2 and negligible mass, how high will the lid rise when the temperature is raised to T = 230°C?
(b) What is the pressure of the gas at 230°C?
Figure: