Assignment:
Q1. Why does cyclopentadiene dimerize so easily and rapidly to dicyclopentadiene? Please explain your answer.
Q2. Why must the distillation head temperature be maintained below 45oC suring the cracking of dicyclopentadiene? In other words, in the cracking of dicyclopentadiene, why is it necessary to distill the product very slowly?
Q3. Draw all the products of the following reactions. And also state whether they will be racemix, and draw its 3D configuration.
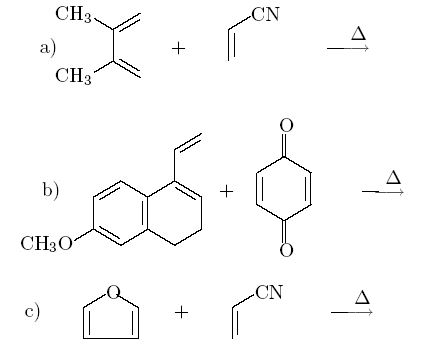
Q4. What starting material would be necessary to prepare the following compound by the Diels-Alder reaction?
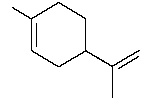
Q5. Draw the diene and dienophile you would use to synthesize each of the following:
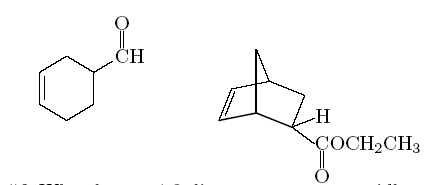
Q6. Why does a 1,3-diene react more rapidly with maleic anhydride that with another molecule of itself?
Q7. Please perform the theoretical yield of
Dicyclopentadiene ↔ Cyclopentadiene. Then cyclopentadiene + maleic anhydride ↔ cis-norbornene-5,6-endo-dicarboxylic anhydride.
Provide complete and step by step solution for the question and show calculations and use formulas.