Assignment:
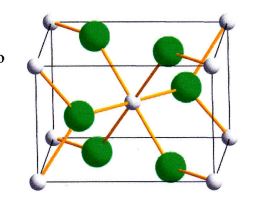
Question 1. A plot ofthe lattice energies for MClz salts for M : Ca to Znand a drawing of the rmit cell are shown to the right. In the elechostatic model for "ionic bonding;'U is proportional to (Z*)(Z-)e2N(r*+ r-) where 7-+ artd Z-are the ion charges, A is the structure (Madelung) constant and r+ * r- are the radii of the ions (see p. 88 of the text for more on this). Since the radius of the cation should decrease systematically as the effective number charge increases on going across the fourth row, one might expect a similar smooth variation in the lattice energy. Clearly, this is not the case. Provide an explanation for the observed variation in lauice energy that accounts for &e fact that the points for CaF, Mn2* andZ,** define anearly straight line thatwould appear to be consistent with the expected gradual decrease in their radii, whereas the other elements have somewhat greater lattice energies than predicted on this basis.
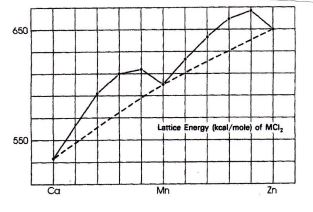
Question 2. Use the experimentally determined values for A" for V2* in solid YClz (9200 cm'; and Ni2* in solidNiClz {6920 crn t) to support your explanation for the phenomena described in problem 3. This requires calculations of LFSE; example problem 20.3 will provide guidance for those who need help. Estimate the actual deviation in lattice energies from the predicted value based on the line connecting the values for Ca2*, Mn2* and Zrf* inthe figure from problem 1.