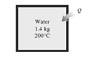
Solve the below problem:
Q: A rigid tank initially contains 1.4-kg saturated liquid water at 200°C. At this state, 25 percent of the volume is occupied by water and the rest by air. Now heat is supplied to the water until the tank contains saturated vapor only. Determine
(a) the volume of the tank,
(b) the final temperature and pressure, and
(c) the internal energy change of the water.