Assignment:
Question 1. This experiment involves a number of redox reactions. They are carried out in the following order of stages.
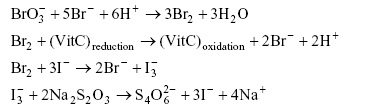
To determine the amount of ascorbic acid in the tablet properly, the limiting reagent condition must be adhered to at each of the stages. Underline the reactant in each of the above reactions that is acting as the limiting reagent.
Question 2. In part I, step 1, it states that " Preparation of 0.1 M Na2S2O3. Boil 150-mL of water for 15 minutes and allow it to cool to room temperature. To this water, add an appropriate amount of Na2S2O3⋅5H2O and 0.20 g of Na2CO3 and stir well. Transfer to a 250-mL bottle and store in the dark. Do not let excess light to reach the solution." While the water is boiling it is unavoidable that some of it will evaporate. How critical is it to maintain the volume of the water to be 150-mL?
Question 3. (Part I, Step 2) Estimate the number of grams of Na2S2O3⋅5H2O that is needed when mixed with 0.20 g of Na2CO3 and dissolved in 150-mL of boiled deionized water will yield a solution of 0.10 M Na2S2O3?
Question 4. (Part I, Step 3) KIO3(s) is soluble in water. Estimate the number of grams of KIO3(s) that is needed to prepare a 100-mL 0.010 M solution?
Question 5. After completing part I what should you do with the unused portion of the sodium thiosulfate solution?
Question 6. (Part II, Step 1) Estimate the number of grams of KBrO3 that is needed to prepare 100-mL 0.0090 M solution?
Question 7. (Part II, Step 2) Suppose the label on the Vitamin C bottle shows that the nominal mass of vitamin C is n-milligram and a tablet weighs x-gram.
a) When the tablet is pulverized in a clean mortar, what is the estimated mass of the powder that should be used so that it has the equivalent of a nominal mass of 100 mg of vitamin C?
b) What is the actual amount of the powder that should be used? Explain.
Please see the manual to answer the questions:
1a. (Part I, Steps 4, 5, & 6) If 13.90-mL of Na2S2O3(aq) are used to titrate with 25.00-mL 0.01022 M KIO3(aq), what is the molarity of the thiosulfate solution?
1b. After 0.1510 g of KBrO3 is weighed and part II step 1 is performed, determine the molar concentration of (not KBrO3)?
1c. Suppose when doing part II step 2, it is found that a randomly selected vitamin C tablet weighs 0.2530 g and the resulting pulverized powder used for the experiment has a mass of 0.1011 g. In part II step 3a it states that "Proceed with a 25-mL aliquot of the sample solution. Add 2.5 g KBr (this amount is excess and need not be exact) and immediately add 25-mL of the standard KBrO3". The reactions are:
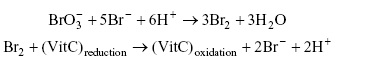
Explain why the 2.5 g of KBr "need not be exact" (in the spreadsheet this is indicated as NNBE) and calculate the number of moles of formed.
1d. Br2 oxidizes the vitamin C according the reaction
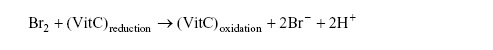
Determine which one is the limiting reagent, vitamin C or Br2?
1e. (Part II, Step 3b) Explain why the 1.5 g KI added to the solution "need not be exact". What is the minimum amount of KI that should be added?
1f. (Part II, steps 3 and 4) If the back titration in part II step 3b takes 10.61-mL of Na2S2O3, how many mg of vitamin C are in the sample used and how many mg of ascorbic acid is in the tablet used?
2. The success of this experiment partly hinges on the "completeness" of a number of the reactions, that is the reactions should proceed almost 100% to the product side. The reactions are,
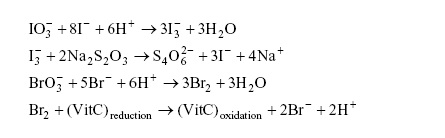
Justify the "completeness" of the third reaction in terms of its equilibrium constant. Show you work in detail and be as quantitative as possible (i.e., calculate the equilibrium constant, K, for this reaction and comment on it magnitude in relationship to "completeness").
Please review the document enclosed here:
Attachment:- Determination of Ascorbic Acid.rar