Discuss the below:
Laws of Thermodynamics
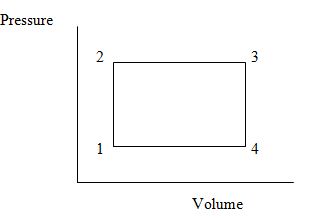
Q: When a gas follows path 123 on the PV diagram below, 418 J of energy flows into the system by heat, and -167 J of work is done on the gas.
a) What is the change in the internal energy of the system?
b) How much energy Q flows into the system if the gas follows path 143? See attached file for diagram. The work done on the gas along this path is -63.0 J.
c) What net work would be done on or by the system if the system followed path 12341? See attached file for diagram.
d) What net work would be done on or by the system if the system followed path 14321? See attached file for diagram.
e) What is the change in internal energy of the system in the processes described in parts c and d?