Assignment:
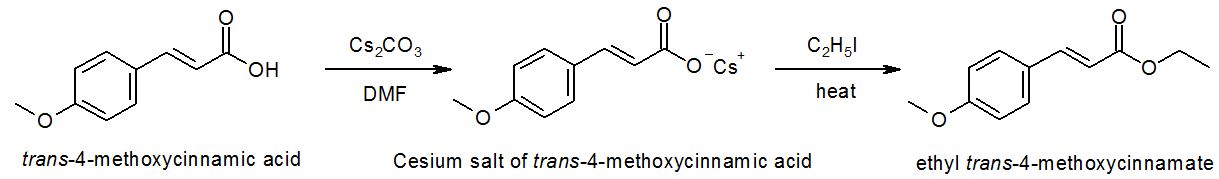
One of the many approaches to ester synthesis is the SN2 reaction of a carboxylate anion with an unhindered alkyl halide. For instance, the reaction of trans-4-methoxycinnamic acid to ethyl trans-4-methoxycinnamate (refer to attached document for mechanism of reaction).
1.Although the sodium salt of trans-4-methoxycinnamic acid is relatively easy to prepare, it is often unreactive in esterification. Instead, cesium carboxylate is much better, with O-alkylation in DMF and heat to yield the ester product. I've considered possible reasons for this... polar aprotic solvents (e.g., DMF) separate the carboxylate anions from each other and the counterion thus promoting O-alkylation, and large alkali cations (e.g., cesium) give more separated ion pairs and more polar bonds again promoting O-alkylation. Is this right? Are there other reasons besides this?
2. In the experimental procedure, trans-4-methoxycinnamic acid, cesium carbonate, and iodoethane are reacted in DMF solvent with nitrogen gas. Why add nitrogen gas?
3. What specific advantages would there be in the reaction of 4-methoxybenzaldehyde with ethyl acetate in sodium ethanoate base over that described in the attached document? ...yield? purity? reaction time? reaction conditions? cost? hazards or health risks? Please elaborate.
4. What is the mechanism by which the cesium carboxylate is formed?
Provide complete and step by step solution for the question and show calculations and use formulas.