Assignment:
Question 1. You are required to prepare working standard solutions of 1.00 x 10^-5, 2.00 x 10^-5, 5.00 X 10^-5, and 1.00 x 10^-4 M glucose from a 0.100 M stock solution. You have available 100 mL volumetric flasks and pipets of 1.00-, 2.00-, 5.00-, and 10.00-mL volume. Outline a procedure for preparing the working standards.
Question 2. A 0.1 M sodium hydroxide solution is to be standardized by titrating primary standard sulfamie acid (N112S03H). What weight of sulfamic acid should be taken so that the volume of NaOH delivered from the buret is about 40 mL?
Question 3. Calcium in a 2.00-g sample is determined by precipitating CaC2O4, dissolv-ing this in acid, and titrating the oxalate with 0:0200 M KMn04. What per-cent of Ca0 is in the sample if 35.6 mL KMn04 is required for titration? (The reaction is 5H2C2O4 + 2MnO4-- + 6H^+ --> 100O2 + 2Mn^2+ + 8H20.)
Question 4. A hydrogen peroxide solution is analyzed by adding a slight excess of stan-dard KNIn04 solution and back-titrating the unreacted K.Mn04 with standard Fe^2+ solution. A 0.587-g sample of the 11202 solution is taken, 25.0 mL of 0.0215' M -KMn04 is added, and the back-titration requires 5.10 ml. of 0.112 M Fe^2+ solution. What is the percent 11202 in the sample? (See Examples 5.25 and 5.29 for the reactions.)
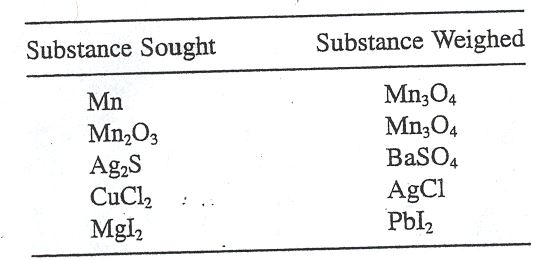
Question 5. Calculate the gravirnetric factors for: