Response to the following problem:
An ideal-gas cycle suggested by A. S. Arrott of British Columbia, Canada, is shown in Fig. , where there are shown on a PV two isothermal curves intersected by an adiabatic curve, referring to 1mol of an ideal monatomic gas. A process takes the gas from the upper intersection point A and expands it isothermally at 600 K to a very special state B. The gas is then put in contact with a low-temperature reservoir at
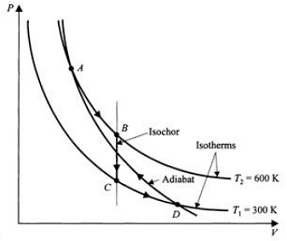
300K so that it cools isochorically to state C. Then, there is a further isothermal expansion from C to the lower intersection point D. The remainder of the zilch cycle is accomplished by an adiabatic compression from D back to A. The isochoric process BC is chosen to satisfy the condition that the net work in the cycle is zero.
(a) Calculate the work WDA.
(b) Calculate the heat QBC.
(c) Calculate the net entropy change of the gas (not the reservoirs) and obtain the relationship
QAB/600K + QCD/300K = 8.64(J/K).
(d) Calculate the work WAB.
(e) Calculate the work WCD.
(I) Calculate the net entropy change of the reservoirs.
(g) Draw the TS diagram.