Detailed Question: Answer these 4 questions using the table attached which we acquired after the practical we had. I also attached the lab handbook with the practical we did (maybe it`s gonna help you out).
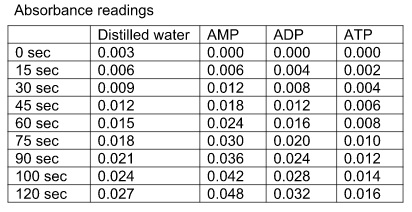
1. Plot one graph of absorbance at 340 nm against time for all four pieces of data
2. Calculate the initial rate (slope of the line) for each enzyme reaction.
3. What effect do AMP, ADP and ATP have on the initial rate of the reaction?
4. Comment on your results and discuss the findings in relation to the theory of this reaction in the citric acid cycle.
Isocitrate dehydrogenase: an important control enzyme
Introduction:
Isocitrate dehydrogenase catalyses the conversion of isocitrate to α-ketoglutarate and carbon dioxide. NAD+ is the electron acceptor in this reaction. The enzyme is a major control point in the citric acid cycle and is affected by the energy charge of the cell. The enzyme is assayed by following the increase in absorbance at 340 nm as NAD+ is reduced to NADH. All the reagents need to be kept on ice as most are not heat stable. The reagents have been made up in Tris buffer. The enzyme preparation is from baker’s yeast.
Method:
1. Set the spectrophotometer to 340 nm and blank with a cuvette full of distilled water by pressing SET REF.
2. Record the absorbance of both pure NAD+ and NADH solutions.
3. Prepare a test tube containing the following reaction mixture (tube 1) and incubate at 37°C for 10 minutes.
NAD+ (2 mM) 1.5 ml
Magnesium chloride (0.1 M) 0.1 ml
Enzyme preparation 0.2 ml
Distilled water 0.2 ml
4. At the same time, in a separate test tube, incubate 1.0 ml of 3 mM sodium isocitrate substrate (tube 2) at 37°C for 10 minutes.
5. After 10 minutes, pour the contents of tube 1 into tube 2 to mix. Add quickly to a 3 ml cuvette, put into the spectrophotometer and press SET REF to zero it. Follow the reaction by recording the absorbance at 340 nm every 15 seconds for two minutes.
6. The initial rate of reaction should give a slope of about 45° when plotted on a graph of absorbance at 340 nm against time.
Effect of altering the energy charge of the reaction mixture
Repeat the assay exactly as above but with the following changes.
a) In tube 1 add 0.2 ml of 3 mM AMP to the enzyme mix, instead of 0.2 ml distilled water.
b) In tube 1 add 0.2 ml of 3 mM ADP to the enzyme mix, instead of 0.2 ml distilled water
c) In tube 1 add 0.2 ml of 3 mM ATP to the enzyme mix, instead of 0.2 ml distilled water.