Solve the below problem:
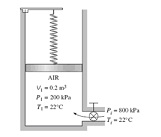
Q: An insulated vertical piston-cylinder device initially contains 0.2 m3 of air at 200 kPa and 22°C. At this state, a linear spring touches the piston but exerts no force on it. The cylinder is connected by a valve to a line that supplies air at 800 kPa and 22°C. The valve is opened, and air from the high-pressure line is allowed to enter the cylinder. The valve is turned off when the pressure inside the cylinder reaches 600 kPa. If the enclosed volume inside the cylinder doubles during this process, determine
(a) The mass of air that entered the cylinder, and
(b) The final temperature of the air inside the cylinder.