Assignment:
Bonding Structures and VSEPR Theory
Help with various molecular geom/chem bonding problems. Any explanations would be greatly appreciated.
1. Fill in the table below for each of the following compounds. The column headings refer to the central atom, which is shown in bold type. (VSEPR Model)
|
Compound
|
Number of Electrons Pairs
|
Arrangement of Pairs
|
Molecular Geometry
|
|
Bonding
|
Lone
|
|
AsCl3
|
|
|
|
|
|
SeCl4
|
|
|
|
|
|
BrF3
|
|
|
|
|
|
ICl4-
|
|
|
|
|
|
NH4+
|
|
|
|
|
2. Determine the molecular geometry for each of the following compounds and use it to explain why each compound has the dipole moment shown. The central atom is shown in bold type.
(Dipole Moments)
a. NF3, nonzero dipole moment
b. BF3, zero dipole moment
c. CF4, zero dipole moment
d. SF4, nonzero dipole moment
e. XeF4, zero dipole moment
3. Identify the hybrid orbitals used by the central atom for each of the following compounds. The central atom is shown in bold type. (Valence Bond Theory)
|
Compound
|
Hybrid Orbitals
|
Compound
|
Hybrid Orbitals
|
|
CCl4
|
|
PF5
|
|
|
SF4
|
|
PCl3
|
|
|
BrF5
|
|
HCN
|
|
|
XeF2
|
|
F2CCF2
|
|
|
H3O+
|
|
Cl2CO
|
|
4. Fill in the table below for the following compound. Consider each carbon atom (numbered in red) a central atom. (Multiple Bonding)
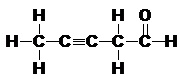
|
Carbon Number
|
Number of Sigma Bonds Around Carbon
|
Number of Pi Bonds Around Carbon
|
Type of Hybrid Orbitals Used by Carbon
|
Bond Angles Around Carbon
|
|
1
|
|
|
|
|
|
2
|
|
|
|
|
|
3
|
|
|
|
|
|
4
|
|
|
|
|
|
5
|
|
|
|
|
5. (Cis-Trans Isomers) Answer the following questions about cis- and trans-1,2-dichloroethene.
a. What is the main structural difference between cis-1,2-dichloroethene and trans-1,2-dichloroethene?
b. Do cis-1,2-dichloroethene and trans-1,2-dichloroethene have the same physical properties? Give an example.
c. Suppose you have a sample of 1,2-dichloroethene. Describe how you could use dipole moments to determine if the sample is the cis isomer or the trans isomer.
d. The cis and trans isomers have geometries that are locked in a rigid structure that prevents easy rotation around the carbon-carbon double bond. What feature of the double bond locks the molecule in either the cis or the trans arrangement?
e. The cis isomer can be converted into the trans isomer, and vice versa, if rotation around the carbon-carbon double bond takes place. Which part of the carbon-carbon double bond in either cis- or trans-1,2-dichloroethene would have to be broken to allow easy rotation around the bond? What could cause this bond to be broken and allow the cis and trans isomers to be interconverted?