Assignment:
Boiling Point, Vaporization and Electronic Configuration
1. Place these hydrocarbons in order of decreasing boiling point
Rank from highest to lowest boiling point. To rank items as equivalent, overlap them.
- paraffin, C36H74 ,
- butane, C4H10 ,
- pentane, C5H12 ,
- neopentane, C5H12 (also known as 2,2-dimethylpropane), and
- nonadecane, C19H40.
2. What is the normal boiling point of this compound? (see attached file for formula)
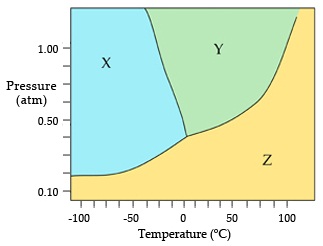
3. The constants for H2O are shown in the attachment.
Part A
How much heat energy, in kilojoules, is required to convert 80.0g of ice at -18.0 degrees C to water at 25.0 degrees C? Express your answer to three significant figures and include the appropriate units.
Part B
How long would it take for 1.50 mol of water at 100.0 degrees C to be converted completely into steam if heat were added at a constant rate of 18.0J/s? Express your answer to three significant figures and include the appropriate units.
4. A 75.0mL sample of water is heated to its boiling point. How much heat (in kJ ) is required to vaporize it? (Assume a density of 1.00 g/mL .)
5. Arrange the liquids in order of decreasing height of the mercury column, designated as h in the image. Rank from largest to smallest height of the mercury column. To rank items as equivalent, overlap them.
CH3CH2CH2CH2-NH2
CH3CH2CHCH3
I
NH2
CH3CH2CH2OCH3
CH3CH2CH2CH2CH3
6. Rank the following elements by electron affinity, from most positive to most negative EA value.
Kr,As,Na,Te,Br
7. Show the complete orbital-filling diagram for N (nitrogen) and Show the complete orbital-filling diagram for S (sulfur). Hund's rule states that if two or more orbitals with the same energy are available, one electron goes in each until all are half full. The electrons in the half-filled orbitals all have the same value of their spin quantum number.
8. Write a complete orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic.
Cd 2+
The electrons occupy different orbitals according to the energy level of each orbital. A single box represents an orbital. The unpaired electron is represented as whereas the paired electrons in the same orbital are represented by two arrows pointing in opposite directions: Check all that apply.
The C atom has two unpaired electrons.
The He atom has two electrons that have parallel spin in its 1s orbital.
The arrangement of the orbitals is the same in a multielectron atom and a single-electron atom.
The He atom has one electron each in 1s and 2s orbitals.
Electrons generally occupy the lowest energy orbital first.
In the Li atom, the 3s , 3p , and 3d orbitals have different energies.
9. For each set of elements represented in this periodic table outline, identify the principal quantum number, n, and the azimuthal quantum number, l , for the highest energy electrons in an atom of one of those elements. Ne, Rn, K, Bh, Ce
10. The black line between elements 56 and 71 in the periodic table shown indicates that in the Lanthanide series elements 57 through 70 are listed below the main table, while in the Actinide series elements 89-102 are listed below the main table. Elements 71 and 103 are listed in main table.Identify the outer electron configuration of each element shown in this periodic table outline.
Cs, Rf, Lr, Xe, Lv, Si, and cd