Assignment:
Question 1. The average speed of nitrogen molecule in air is about 6.70x10^2m/s, and its mass is 4.68x10^(-26) kg
a) If it takes 3.00x10^-13s for a nitrogen molecule to hit a wall and rebound with the same speed but opposite direction, what is the average acceleration of the molecule during this time interval
b)What average force does the molecule exert on the wall?
Question 2. A bag of cement weigh 325N hangs in equilibrium from three wires as suggested of the picture below. Two of the wires make angle φ1=60 degree, φ2=25 degree with the horizontal. Find the tension T1, T2 and T3 of the wires.
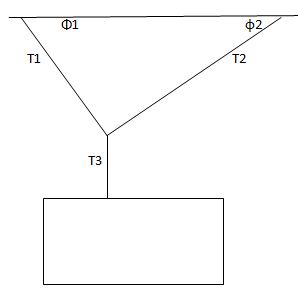
Question 3. Based of the picture above to. A bag of cement weigh Fg hang in equilibrium from three wires as shown. Two of the wires make angles φ1, φ2 with the horizontal. Show that the tension on the left hand wire is
T1= (Fg.cosφ2)/(sin(φ1+φ2))