Assignment:
Uranium, symbol U, is one of the rarer elements in the earth’s crust, where it occurs mainly as the oxide uraninite (U3 08 ). In 1938, Otto Hahn and Fritz Strassmann showed unambiguously that new, lighter, elements are formed when Uranium is bombarded with neutrons, a process now referred to as a nuclear fission. The Second World War provided the stimulus to exploit the fission or uranium to make weapons, and the process Is now used as an energy source to fuel nuclear power plants.
(A) The distribution of uranium deposits is very uneven across the world. Table 2 shows recent estimates of each continents share of the total ‘reasonably assured’ Uranium resources (i.e. Identified material that could be mined), expressed in terms of the mass of uranium available. (Note‘t’ denotes tonne, i.e. 103 kg.)
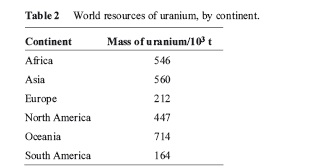
(i)China has 6.8% of Asia’s Uranium resources. Calculate the mass of uranium in china’s resources, expressing your answer in Kg in scientific notation. Show your working
(ii)Express the ratio of the North America’s uranium resources to total world resources in the form 1: x show you’re working.
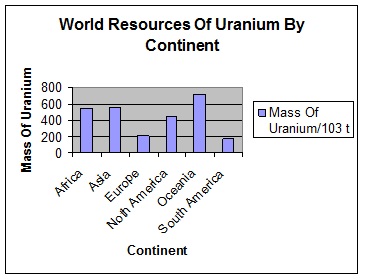
(iii) Plot date shown in table 2 as a histogram, using the graph paper on page 29 of booklet. Draw your histogram by hand, remembering to include a title and to label the axes suitably Hand Drawn Histogram.
(B) Typically, a low-grade uranium ore yields only 0.2% of it’s mass as uranite, U3 08; the rest is unwanted rock spoil
(i)Calculate the percentage by mass of uranium in uraninite. Show your working. (The relative atomic masses are: Uranium 238; Oxygen 16.0)
(ii) During its lifetime ( about 30 years ), a typical nuclear reactor in the civilian sect or requires around 5 x 103 tonnes of uranium. Calculate what mass of low –grade uranium ore has o be mined to meet this requirement, giving your answer in Kg in scientific notation. Show your working.
(c) Uranium has three naturally occurring isotopes: 238u, 235u and 234u Natural uranium is composed of approximately 99.284% 238u, 0.711% 235u and only 0.0055 %/234u.
However the fuel in most of the world’s reactors is solid uranium dioxide, uo2 in which the 238u content has been increased to about 3 %. This ‘enrichment’ Process is carried out with the compound UF6 is reacted with hydrogen fluoride, which is given off a gas. Equation A1 is an unbalanced form of the chemical equation for this reaction.
UF6+ H20+ H2 ---> UO2 + HF
Write a balanced equation for the reaction using the lowest possible whole – number coefficients, and including the state of each compound.
Provide complete and step by step solution for the question and show calculations and use formulas.