Assignment:
Q1. Which one of the following is the least effective method for the synthesis of the compound shown?
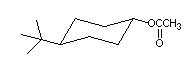
a. treat trans-4-tert-butylcyclohexanol with sodium acetate
b. combine trans-4-tert-butylcyclohexanol and acetic anhydride in the presence of pyridine
c. combine trans-4-tert-butylcyclohexanol and acetyl chloride in the presence of pyridine
d. dissolve trans-4-tert-butylcyclohexanol in acetic acid containing a few drops of H2SO4
Q2. The behaviors of acyl chlorides and aldehydes in a reaction with a nucleophile are different because:
a. The carbonyl carbon of the aldehyde is more positive.
b. The bulky chlorine sterically hinders nucleophilic attack at the carbonyl group – I’m totally guessing
c. The acyl chloride cannot form a tetrahedral intermediate
d. There is a good leaving group in the acyl chloride
e. The aldehyde has a readily oxidizable hydrogen
Q3. What is the product of the following reaction?
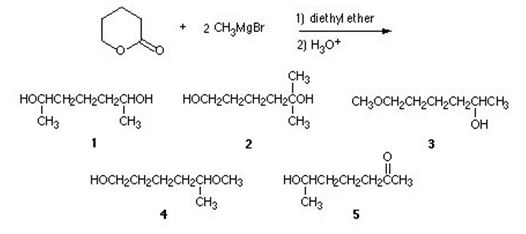
a. 1
b. 2
c. 3
d. 4
e. 5
Q4. The tetrahedral intermediate in the Fischer esterification of formic acid (HCO2H) with 1-butanol has the structure:
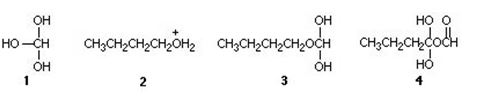
a. 1
b. 2
c. 3
d. 4
Q5. For the reaction performed in basic solution, which one of the following tetrahedral intermediates is most likely to dissociate to an ester?
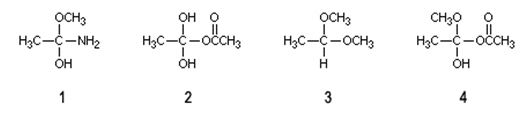
a. 1
b. 2
c. 3
d. 4
Q6. Reaction of benzoic acid with CH318OH in the presence of an acid catalyst yields:

a. 1
b. 2
c. 3
d. 4
Q7. Which of the following species is not an intermediate in the generally accepted mechanism for the reaction shown?
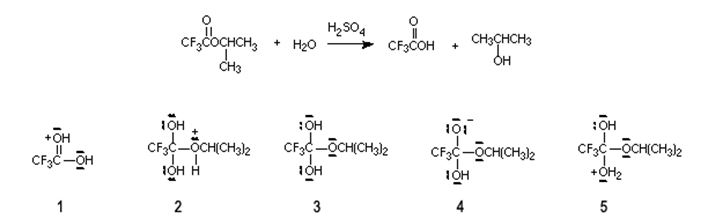
a. 1
b. 2
c. 3
d. 4
e. 5
Q8. Which one of the following is the most effective method for the synthesis of the compound shown?
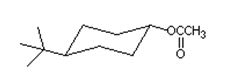
a. treat trans-4-tert-butylcyclohexanol with sodium acetate
b. treat trans-1-bromo-4-tert-butylcyclohexane with sodium acetate
c. combine trans-4-tert-butylcyclohexanol and acetic anhydride in the presence of pyridine
d. combine cis-4-tert-butylcyclohexanol and acetyl chloride in the presence of pyridine
Q9. Hydrolysis of ethyl acetate in aqueous base proceeds by attack of:
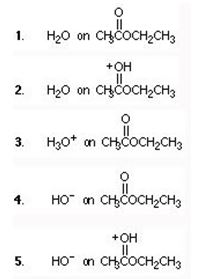
a. 1
b. 2
c. 3
d. 4
e. 5
Q10. Which structure corresponds to the tetrahedral intermediate formed during the reaction of an ester with an amine?
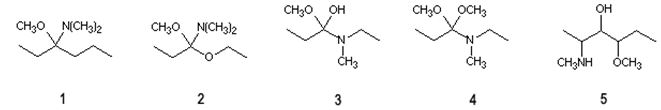
a. 1
b. 2
c. 3
d. 4
e. 5