Discussion:
Simple Ways of Expanding
Q: A plot of pressure as a function of volume is known as a pV diagram. pV diagrams are often used in analyzing thermodynamic processes.
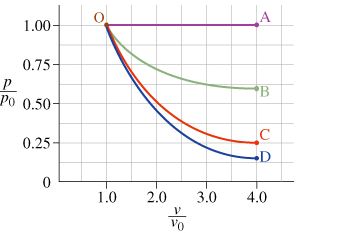
Consider an ideal gas that starts in state O, as indicated in the diagram. Your task is to describe how the gas proceeds to one of four different states, along the different curves indicated. Although there are an infinite number of such curves, several are particularly simple because one quantity or another does not change:
• Adiabatic ΔQ: No heat is added or subtracted.
• Isothermal ΔT: The temperature does not change. (The prefix "iso" means equal or alike.)
• Isobaric Δp: The pressure does not change. (A barometer measures the pressure.)
• Isochoric ΔV: The volume does not change. (This process is infrequently used.)
The key idea in determining which of these processes is occurring from a pV plot is to recall that an ideal gas must obey the ideal gas equation of state: pV=NkBT
(where the constant kB is the Boltzmann constant, which has the value 1.381*10^-23 J/k in SI units). Generally , the number of gas particles, is held constant, so you can determine what happens to at various points along the curve on the pV diagram.
Note that in this problem, as is usually assumed, the processes happen slowly enough that the gas remains in equilibrium without hot spots, without propagating pressure waves from a rapid change in volume, or without involving similar nonequilibrium phenomena. Indeed, the word "adiabatic" is often used by scientists to describe a process that happens slowly and smoothly without irreversible changes in the system.
Part A
What type of process does curve OA represent?
A) adiabatic
B) isobaric
C) isochoric
D) isothermal
Part B
What type of process does curve OC represent?
A) adiabatic
B) isobaric
C) isochoric
D) isothermal
Part C
The pressure of the system in state B is ________ the pressure of the system in state O.
A) greater than
B) less than
C) equal to
Part D
The work done by the system is ________.
A) greater than zero
B) less than zero
C) equal to zero
Part E
The temperature of the system in state B is ________ the temperature of the system in state O.
A) greater than
B) less than
C) equal to
Part F
The internal energy of the system in state B is ________ the internal energy of the system in state O.
A) greater than
B) less than
C) equal to