Assignment:
Q1.
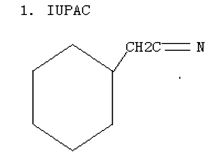
Q2. Even through the para position is one carbon father from the coarboxyl group than the meta position, p-cyanobenzoic acid is more acidic than m-cyanobenzoic acid. Explain the differences in acidity between p-cyanobenzoic acid and m-cyanobenzoic acid.
Q3. Explain the differences in acidity between p-methoxybenzoic and m-methoxybenzoic acid.
Q4. Write the complete stepwise mechanism for the basic hydrolysis of acetamide, shown below. Show all electron flow with arrows and draw all intermediate structures.

Q5. Consider structures answer questions. Indicate acidic hydrogen molecules. Rank molecules order increasing acidity (least acidic).
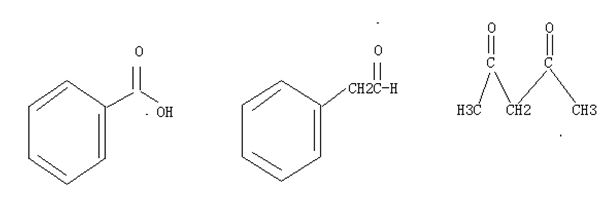
Q6. What order decreasing reactivity nucleophilic acyl substitutions carboxylic acid derivatives? (most relative first)
A.
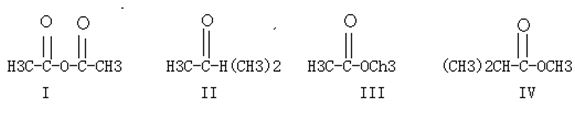
B.
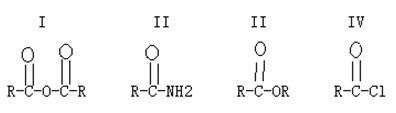
Provide complete and step by step solution for the question and show calculations and use formulas.