Problem 1
The micellization of surfactant can be represented by a stepwise association process given by the equilibrium reaction below:
For a micellization process with identical equilibrium constant for each step, show that the C, M, W and the polydispersity index are given by the expression below:
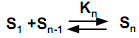
(Note: please refer to your notes for more information)
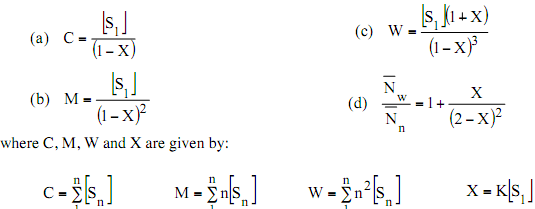
Problem 2
(a) The following surface tension were measured for aqueous solutions of the nonionic surfactant CH3(CH2)9(OCH2CH2)5OH at 25oC

Determine the critical micelle concentration and calculate the area occupied by each adsorbed surfactant molecule at the critical micelle concentration.
(b) The critical micelle concentration of nonionic surfactant in water varies with temperature as follows:

Determine an enthalpy of micellization according to the phase separation model.