Question 1: Draw the two singly occupied molecular orbital of the cycloheptatrienyl anion, [C7H7]–. Sketch alongside each of such singly occupied MOs the metal orbital with the right symmetry for a bonding interaction. Suppose the z-axis runs via the metal centre and the centre of the ring of carbon atoms.
Question 2: N-heterocyclic carbenes are very poor π-acceptors and are less tunable both sterically and electronically than phosphines. Describe the reasons for these observations.
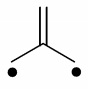
Question 3: Trimethylenemethane (below) is a planar diradical and extremely unstable in the Free State. Though, it is stable as a ligand for transition metals and all four carbon atoms bind to the metal. Draw possible resonance forms for this ligand binding to a metal and recommend how it might be distorted from planarity on binding. How many electrons does it donate to the metal? In an M(CO)3(η4-trimethylenemethane) complex, determine the oxidation state of M?
Question 4: Cobaltocene, Cp2Co, is stable at room temperature; however the rhodium analogue dimerizes to give the compound Rh2C20H20. Draw two possible structures for the product; both must obey the 18 electron rule.