A heater (heat source temperature = 527 K) and turbine are connected in series as shown below:
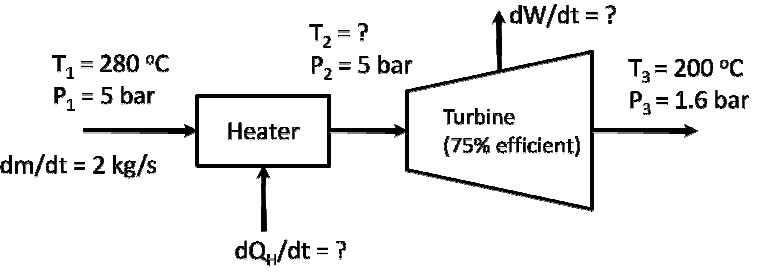
a. Calculate the entropy generation if the process stream is steam.
b. Calculate the entropy generation if the process stream is air. You can treat air as an ideal. Calculate the entropy generation if the process stream is air. You can treat air as an ideal Calculate the entropy generation if the process stream is air. You can treat air as an ideal gas with Cp = 27 J/mol/K and molecular weight of 29 g/mol.